
Modern clinical sample processing demands speed, precision, and scalability. Our custom-built end-to-end molecular assay work cell design meets these challenges head-on with a sophisticated dual-robot system that seamlessly integrates DNA extraction and qPCR analysis into a single automated workflow (Fig. 1).

Figure 1. HighRes Automated Bioanalytical Testing System. The dimensions are only 10' x 20' x 7.8' (w x l x h). This design prioritizes maximizing throughput and walkaway time while minimizing the physical footprint required to achieve the system's objectives.
Bioanalytical Testing System Design
The work cell features a thoughtfully designed two-robot configuration, each serving specialized functions while sharing critical infrastructure (Fig. 2).
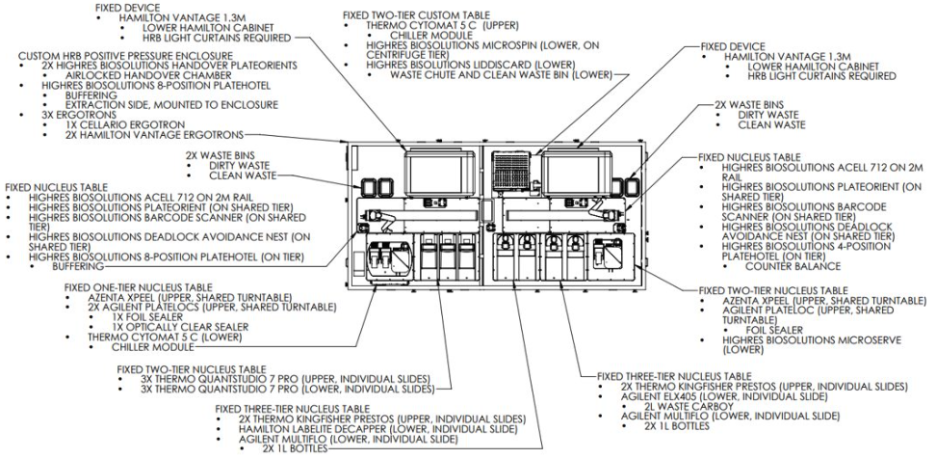
- Robot #1 (Right Envelope) handles all extraction-related operations, providing dedicated access to sample preparation and purification devices.
- Robot #2 (Left Envelope) manages qPCR operations while also controlling shared resources including storage systems, seal management, and centrifugation equipment.
Two HighRes PlateOrients serve as intelligent handover points between the robot envelopes, enabling smooth plate transfers and efficient workflow orchestration. Each envelope includes its own dedicated liquid handler, optimizing parallel processing capabilities.
For the End-to-End Molecular Assay System, the dimensions are only 10' x 20' x 7.8' (w x l x h). This design clearly prioritizes maximizing throughput and walkaway time while minimizing the physical footprint required to achieve the system's objectives (Fig. 3).
(A) 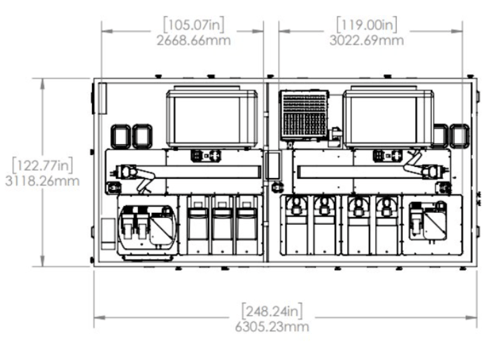
(B) 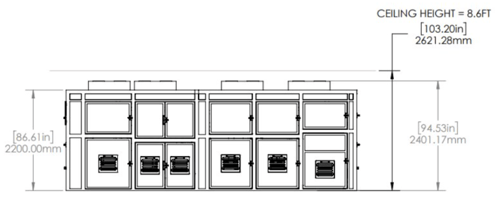
Figure 3. For the End-to-End Molecular Assay System, the dimensions are 10' x 20' x 7.8' (w x l x h). (A) The top-down view of the system; (B) the lateral view of the system.
The entire system operates under a single CellarioScheduler instance, offering the flexibility to execute the complete molecular assay as either one integrated protocol or multiple coordinated protocols (Fig. 4).
Unlike traditional schedulers that require complex programming and custom scripting to coordinate multi-robot workflows, CellarioScheduler's intuitive protocol design enables straightforward configuration of even sophisticated automation sequences (Fig. 4). This architecture supports true parallel processing of multiple orders simultaneously, with the scheduler handling all inter-instrument timing, resource allocation, and workflow dependencies through its user-friendly interface.
The featured instruments in this system are:
- 2x HighRes ACell 712 on 2M Rail
- HighRes PlateOrient
- HighRes Barcode Scanner
- HighRes Deadlock Avoidance Nest
- HighRes MicroSpin
- HighRes LidDiscard
- HighRes 8-Position PlateHotel
- HighRes MicroServe
- HighRes Enclosure with Envelope Divider
- Agilent ELx405 Select Deep Well Washer
- 2x Agilent MultiFlo FX
- 3x Agilent PlateLoc
- 2x Peelable Foil Sealer
- 1x Optically Clear Sealer
- 2x Azenta Automated Plate Peeler (formerly Brooks XPEEL)
- 1x Hamilton LabElite
- 2x Hamilton VANTAGE
- 2x Thermo Cytomat 5 C
- 4x Thermo KingFisher Presto
- 6x Thermo QuantStudio 7 Pro
- 3x HighRes Nucleus Table
- 2x HighRes Two-Tier Nucleus Table
- 2x HighRes Three-Tier Nucleus Table
- 1x HighRes Two-Tier Custom Table
- 2x Solids Waste Bin, Clean Materials
- 2x Solids Waste Bin, Biohazardous Materials
DNA Extraction: Optimized for Automation
DNA Extraction in Clinical Sample Processing
DNA extraction is a critical first step in molecular assays for clinical diagnostics, as it isolates and purifies nucleic acids from complex biological samples such as blood, tissue, or swabs. The process typically involves cell lysis to release DNA, followed by separation of DNA from proteins, lipids, and other cellular debris.
The quality and quantity of extracted DNA directly impact downstream assay performance, making quality assurance steps essential for reliable clinical results. Automated platforms can process multiple samples simultaneously with standardized protocols, ensuring reproducibility across batches, a crucial requirement for clinical validation and regulatory compliance.
KingFisher Presto Selection
While some may prefer KingFisher Apex, we recommend the Thermo KingFisher Presto for integrated automation environments.
Here's why:
The Apex, though excellent as a benchtop device, requires manual door operation, a significant limitation in fully automated systems. The Presto eliminates this limitation entirely.
The Presto's turntable design offers substantial throughput advantages. Plates can be rapidly streamed on and off the device, dramatically improving utilization rates. Our custom Cellario hook enables the loading of subsequent wash or collection plates while processing is actively underway, a game-changer for high-throughput operations.
UV Sterilization Option: While the Presto doesn't include native UV sterilization, we can provide a custom sub-enclosure that accommodates UV-sterilizing light systems if this capability is required.
Scalability: The system design accommodates up to four KingFisher devices, though the actual number needed will depend on your specific processing step durations and throughput targets.
Supporting Infrastructure
The extraction workflow is supported by a comprehensive suite of automation devices:
- Cytomat 5 C automated refrigeration for sample tube rack storage
- AmbiStore ambient carousel for consumables (tips, combs, wash plates, collection plates)
- LidDiscard for automated tip box lid removal
- LabElite cap handling for tube rack cap management during transfers
- MultiFlo FX peristaltic dispensers for precise buffer addition (lysis, wash, elution)
- ELx405 deep-well plate washer for post-KingFisher wash buffer removal
- Bioshake orbital shakers for sample-bead binding
- MicroSpin centrifuge for separation steps
- PlateLoc and Azenta Automated Plate Seal Remover (formerly XPeel) devices for automated sealing and de-sealing
Liquid Handler Versatility
The VANTAGE liquid handler serves as the workhorse for multiple critical operations:
- Sample transfer from tube racks to assay labware
- Extraction control addition
- Magnetic bead dispensing
- Inter-plate sample transfers
- Final purified sample transfer to storage labware
Purified samples are ultimately sealed and stored in deep-well plates within the automated refrigerator, maintaining sample integrity until qPCR processing.
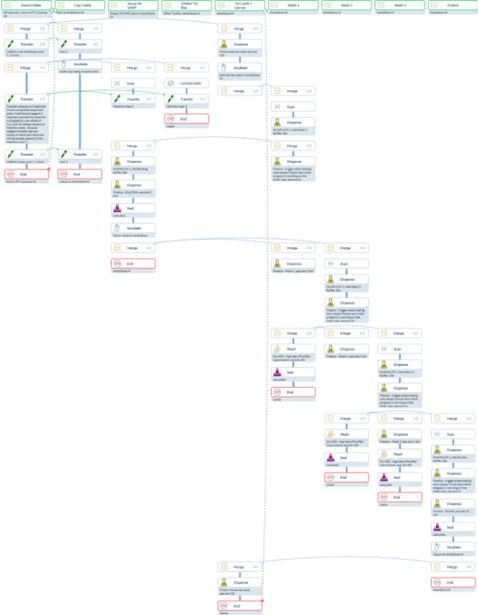
Figure 4. Simulated CellarioScheduler Protocol Design for Extraction in the End-to-End Molecular Work Cell
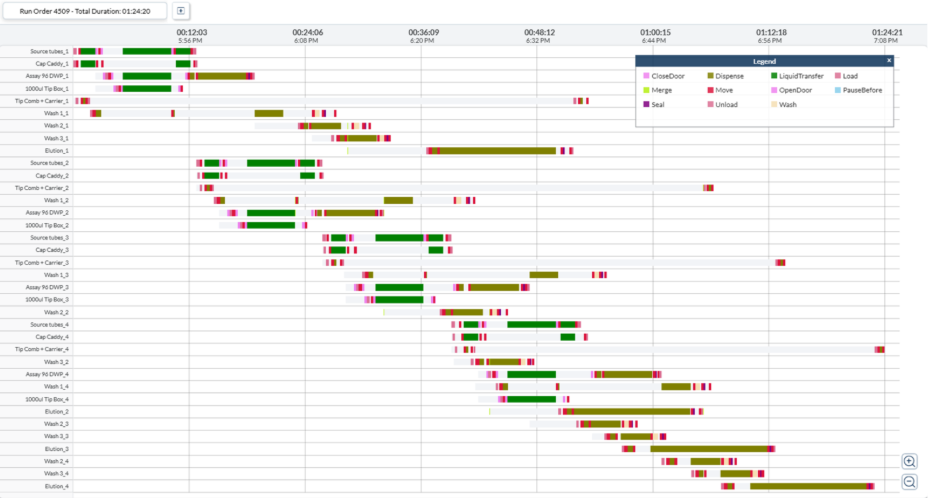
Figure 5. Simulated Gantt Chart for Extraction in the End-to-End Molecular Work Cell
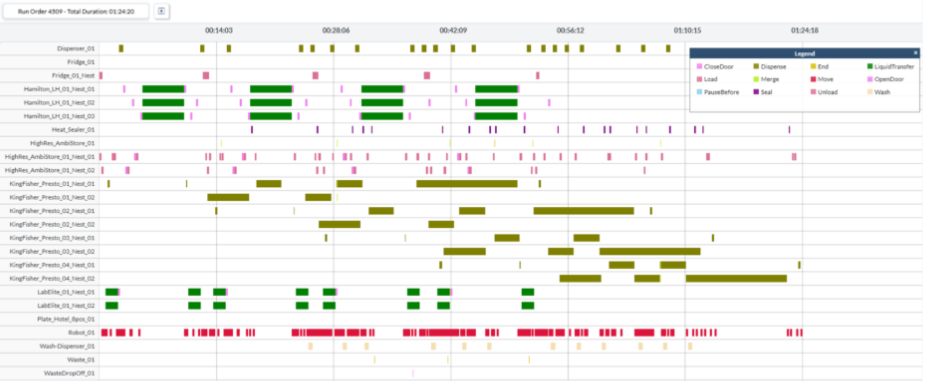
Figure 6. Simulated Resource Utilization Chart for Extraction in the End-to-End Molecular Work Cell
qPCR: High-Throughput Analysis
qPCR in Clinical Molecular Assays
Quantitative polymerase chain reaction (qPCR), also known as real-time PCR (rtPCR), has become the gold standard for detecting and quantifying specific DNA or RNA sequences in clinical diagnostics. This technique amplifies target nucleic acid sequences exponentially while monitoring fluorescent signals in real-time, allowing for both identification and quantification of pathogens, genetic mutations, or gene expression levels.
In clinical settings, qPCR is widely used for infectious disease testing, oncology diagnostics, pharmacogenomics, and genetic disorder screening due to its exceptional sensitivity, specificity, and dynamic range. The assay combines purified DNA or cDNA with sequence-specific primers, a DNA polymerase, and fluorescent reporter molecules (either intercalating dyes or sequence-specific probes) in a master mix that undergoes repeated cycles of heating and cooling.
Modern qPCR instruments can process samples in 96-well or 384-well formats, enabling high-throughput screening essential for clinical laboratories managing large sample volumes. The ability to multiplex (i.e., detecting multiple targets simultaneously in a single reaction) further enhances efficiency and reduces sample consumption, making qPCR an indispensable tool in contemporary molecular diagnostics.
QuantStudio 7 Pro Configuration
The qPCR module utilizes Thermo QuantStudio 7 Pro technology, with each device configured for either 96-well or 384-well formats. Our proposal assumes 384-well operation, which offers significant advantages.
Important Considerations for 96-Well Format:
Should 96-well format be required instead, several important factors must be addressed:
- Half-skirted labware requirements (wells protrude beyond SBS footprint)
- Potential need for carrier plates in certain workflow steps
- Compatibility challenges with oversized carrier plates in tight plate nests
- Labware sticking issues in serial storage devices (necessitating random-access storage with pre-mounted carriers)
- Static nest requirements for labware dismounting
- Potentially twelve additional QuantStudio devices to maintain throughput
- Increased storage capacity for extended walkaway times
Master Mix Management Strategy
Master mix contains temperature-sensitive enzymes and reagents that degrade when exposed to ambient conditions for extended periods. We've designed an intelligent master mix workflow that optimizes both efficiency and reagent stability:
In a separate pre-processing protocol, empty PCR plates are streamed through a master mix dispensing step, currently allocated to the liquid handler, though a dedicated device like the Formulatrix Mantis could be integrated to prevent liquid handler bottlenecks if throughput demands increase. This pre-processing approach minimizes reagent exposure by limiting bulk master mix to the system only during the brief plate-filling step. Once plates are filled, bulk master mix can be immediately removed and returned to refrigerated storage, while the sealed, pre-filled plates are stored in the automated fridge until needed. This strategy maintains reagent integrity by keeping master mix chilled until immediately before use, with plates pulled on-demand as the qPCR workflow requires them.
Pre-filled plates are then sealed and refrigerated until needed. This approach minimizes the time bulk master mixes spend on the work cell while keeping reagents properly chilled until immediately before use.
The alternative approach, dispensing master mix "just in time" during active processing, would require bulk reagents to remain loaded on the system throughout extended run times between qPCR reads. For reagents with stability concerns at room temperature, prolonged ambient exposure risks enzymatic degradation, reduced assay performance, and potential batch-to-batch variability.
By front-loading the dispensing step and utilizing cold storage, this approach optimizes both reagent stability and system efficiency while reducing the risk of compromised assay results due to reagent degradation.
PCR Setup and Processing
The liquid handler orchestrates PCR plate setup from purified samples. In a typical workflow, one 96-format purified sample plate is aliquoted across four 384-format PCR plates, each destined for one of the four qPCR devices.
This design includes built-in resilience: if a qPCR device experiences downtime, prepared PCR plates can be temporarily stored in the automated fridge until capacity becomes available. Meanwhile, source plates with remaining purified samples are sealed and returned to refrigerated storage.
Sealing Options
The current design includes one heat sealer configured for optical seal material—the standard for qPCR assays. A second sealer for peelable foil seals can be added upon request if dual sealing capabilities are needed.
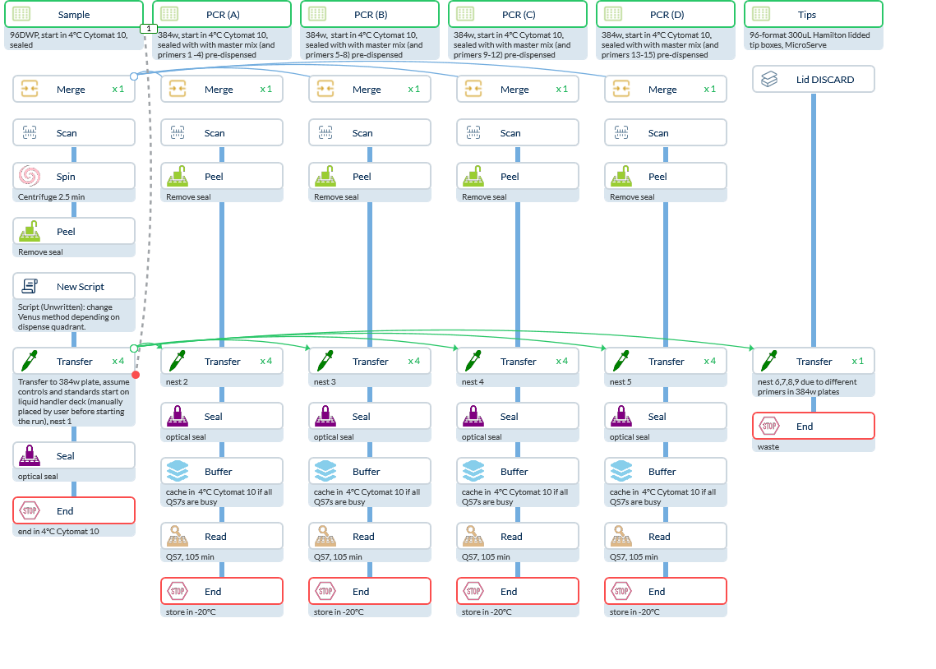
Figure 7. CellarioScheduler Protocol Design for qPCR in the End-to-End Molecular Work Cell
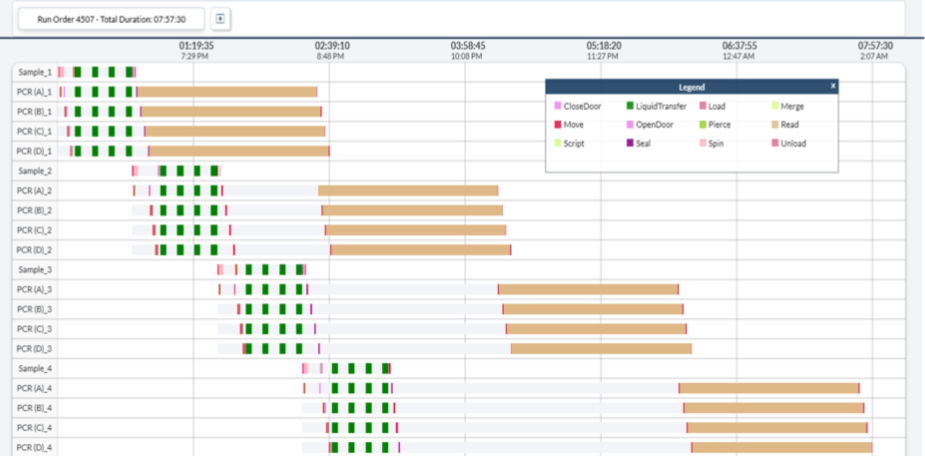
Figure 8. Simulated Gantt Chart for qPCR in the End-to-End Molecular Work Cells
System Specifications
The work cell's physical footprint and device layout have been optimized for both accessibility and workflow efficiency. Detailed dimensional drawings and 3D visualizations demonstrate the careful consideration given to maintenance access, plate routing, and ergonomic operator interfaces.
Protocol Development and Simulation
Based on the provided standard operating procedures, we've developed comprehensive Cellario protocols for both extraction and qPCR assays (Fig. 4; Fig. 7). Where information gaps existed, we've made reasonable operational and timing assumptions that can be refined collaboratively before system testing.
Detailed simulation results, including Gantt charts for workflow visualization, validate the system's throughput capabilities and identify optimization opportunities (Fig. 5; Fig. 8). Note: tip thread operations are excluded from qPCR simulation charts for clarity.
Moving Forward
This end-to-end molecular assay work cell represents a sophisticated solution for high-throughput clinical sample processing. The dual-robot architecture, intelligent storage management, and thoughtful device selection combine to create a system that's both powerful and flexible.
We're ready to work closely with your team to finalize protocol designs, refine operational parameters, and ensure the system meets your specific throughput and quality objectives.
For more information about system capabilities, customization options, or to schedule a detailed technical review, tell us about your project today!
